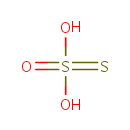
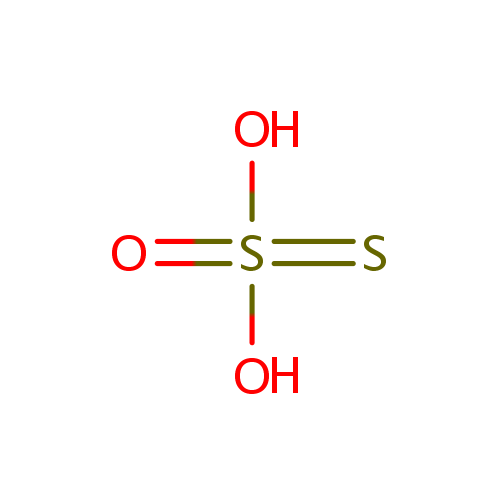
Thiosulfate (PAMDB000108)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000108 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Thiosulfate | ||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Thiosulfate occurs naturally in hot springs and geysers, and is produced by certain biochemical processes. Thiosulfate is an intermediate in several biochemical pathways, including the synthesis of L-cysteine. Thiosulfate is manufactured by some cells by oxidation of elemental sulfur and by degradation of L-cysteine. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | O3S2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 112.128 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 111.928885246 | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | DHCDFWKWKRSZHF-UHFFFAOYSA-L | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/H2O3S2/c1-5(2,3)4/h(H2,1,2,3,4)/p-2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 14383-50-7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | dihydroxy-1???disulfen-1-one | ||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | thiosulfuric acid | ||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [O-]S([S-])(=O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of inorganic compounds known as non-metal thiosulfates. These are inorganic non-metallic compoundscontaining a thiosulfate as its largest oxoanion. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous non-metal compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Non-metal oxoanionic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Non-metal thiosulfates | ||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Non-metal thiosulfates | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 48 °C | ||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Adenosine triphosphate + Water + Thiosulfate > ADP + Hydrogen ion + Phosphate + Thiosulfate Adenosine triphosphate + Water + Thiosulfate > ADP + Hydrogen ion + Phosphate + Thiosulfate Hydrogen cyanide + Thiosulfate > Hydrogen ion + Sulfite + Thiocyanate Thiosulfate + Cyanide <> Sulfite + Thiocyanate 3-Mercaptopyruvic acid + Sulfite <> Thiosulfate + Pyruvic acid O-Acetylserine + Thiosulfate <> Cysteine-S-sulfate + Acetic acid O-Acetylserine + Thiosulfate + Thioredoxin + Hydrogen ion <> L-Cysteine + Sulfite + Thioredoxin disulfide + Acetic acid -->-->Thiosulfate + Hydrogen cyanide > Sulfite + Thiocyanate Cyanide + Thiosulfate + Cyanide + Thiosulfate > Thiocyanate + Sulfite + Hydrogen ion + Thiocyanate + Sulfite O-Acetylserine + Thiosulfate + Thiosulfate > Cysteine-S-sulfate + Acetic acid | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Serikova, E. A.; Racheva, I. V. Method for producing sodium thiosulfate. U.S.S.R. (1986), CODEN: URXXAF SU 1279954 A1 19861230 Patent written in Russian. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||