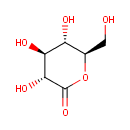
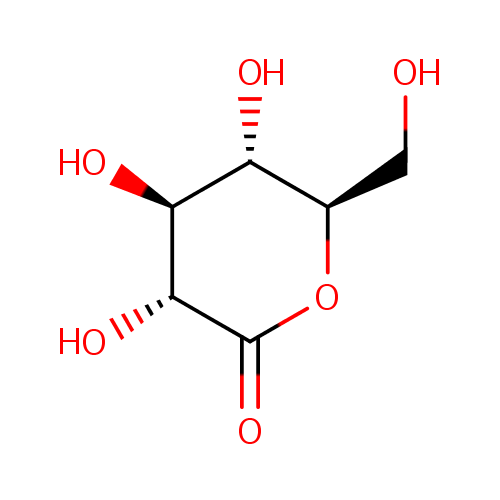
| Synonyms: | - δ-gluconolactone
- 1,5-D-Gluconolactone
- 1,5-delta-Gluconolactone
- 1,5-Gluconolactone
- 1,5-δ-Gluconolactone
- 3,4,5-Trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-one
- 3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-one
- b-glucono-1,5-Lactone
- Beta-Glucono-1,5-Lactone
- D(+)-Gluconate g-lactone
- D(+)-Gluconate gamma-lactone
- D(+)-Gluconate γ-lactone
- D(+)-Gluconic acid g-lactone
- D(+)-Gluconic acid gamma-lactone
- D(+)-Gluconic acid γ-lactone
- D-(+)-Gluconate D-lactone
- D-(+)-Gluconate-delta lactone
- D-(+)-Gluconate-δ lactone
- D-(+)-Gluconic acid D-lactone
- D-(+)-Gluconic acid-delta lactone
- D-(+)-Gluconic acid-δ lactone
- D-Aldonolactone
- D-delta-Gluconolactone
- D-Gluconate 1,5-lactone
- D-Gluconate D-lactone
- D-Gluconate delta-lactone
- D-Gluconate lactone
- D-Gluconate δ-lactone
- D-Gluconate-1,5-lactone
- D-Gluconate-delta-lactone
- D-Gluconate-δ-lactone
- D-Gluconic acid 1,5-lactone
- D-Gluconic acid D-lactone
- D-Gluconic acid delta-lactone
- D-Gluconic acid lactone
- D-Gluconic acid δ-lactone
- D-Gluconic acid-1,5-lactone
- D-Gluconic acid-delta-lactone
- D-Gluconic acid-δ-lactone
- D-Gluconic delta-lactone
- D-Gluconic δ-lactone
- D-glucono-δ-lactone
- D-Glucono-1,5-lactone
- D-Glucono-D-lactone
- D-Glucono-delta-lactone
- D-glucono-δ-Lactone
- D-Gluconolactone
- D-δ-Gluconolactone
- Delta-(+)-Gluconate D-lactone
- Delta-(+)-Gluconate-delta lactone
- Delta-(+)-Gluconic acid D-lactone
- Delta-(+)-Gluconic acid-delta lactone
- Delta-Aldonolactone
- Delta-D-Gluconolactone
- Delta-delta-Gluconolactone
- Delta-Gluconate 1,5-lactone
- Delta-Gluconate D-lactone
- Delta-Gluconate delta-lactone
- Delta-Gluconate lactone
- Delta-Gluconate-1,5-lactone
- delta-Gluconate-delta-lactone
- Delta-Gluconic acid 1,5-lactone
- Delta-Gluconic acid D-lactone
- Delta-Gluconic acid delta-lactone
- Delta-Gluconic acid lactone
- Delta-Gluconic acid-1,5-lactone
- Delta-Gluconic acid-delta-lactone
- Delta-Gluconic delta-lactone
- Delta-Glucono-1,5-lactone
- Delta-Glucono-delta-lactone
- Delta-Gluconolactone
- Fujiglucon
- g-Gluconolactone
- Gamma-Gluconolactone
- Glucarolactone
- Gluconate lactone
- Gluconate, lactone
- Gluconic acid lactone
- Gluconic acid, lactone
- Gluconic delta-lactone
- Gluconic lactone
- Gluconic δ-lactone
- Glucono 1,5-lactone
- Glucono delta lactone
- Glucono delta-lactone
- glucono g-Lactone
- Glucono gamma-lactone
- glucono γ-Lactone
- glucono δ Lactone
- glucono δ-Lactone
- Glucono-δ-lactone
- Glucono-1,5-lactone
- Glucono-delta-lactone
- glucono-δ-Lactone
- Gluconolactone
- β-glucono-1,5-Lactone
- γ-Gluconolactone
- δ-(+)-Gluconate D-lactone
- δ-(+)-Gluconate-δ lactone
- δ-(+)-Gluconic acid D-lactone
- δ-(+)-Gluconic acid-δ lactone
- δ-Aldonolactone
- δ-D-Gluconolactone
- δ-Gluconate 1,5-lactone
- δ-Gluconate D-lactone
- δ-Gluconate lactone
- δ-Gluconate δ-lactone
- δ-Gluconate-1,5-lactone
- δ-Gluconate-δ-lactone
- δ-Gluconic acid 1,5-lactone
- δ-Gluconic acid D-lactone
- δ-Gluconic acid lactone
- δ-Gluconic acid δ-lactone
- δ-Gluconic acid-1,5-lactone
- δ-Gluconic acid-δ-lactone
- δ-Gluconic δ-lactone
- δ-glucono-1,5-Lactone
- δ-glucono-δ-Lactone
- δ-Gluconolactone
- δ-δ-Gluconolactone
|
|---|
| References: |
- Bennett, B. D., Kimball, E. H., Gao, M., Osterhout, R., Van Dien, S. J., Rabinowitz, J. D. (2009). "Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli." Nat Chem Biol 5:593-599. Pubmed: 19561621
- Friedrich T, Strohdeicher M, Hofhaus G, Preis D, Sahm H, Weiss H: The same domain motif for ubiquinone reduction in mitochondrial or chloroplast NADH dehydrogenase and bacterial glucose dehydrogenase. FEBS Lett. 1990 Jun 4;265(1-2):37-40. Pubmed: 2142103
- Harkness RA, Purkiss P, Duffy S, Chalmers RA, Jones M: The effects of fetal energy depletion on amniotic fluid concentrations of amino acids, organic acids and related metabolites. J Inherit Metab Dis. 1988;11(1):103-13. Pubmed: 3128683
- Hunt MJ, Barnetson RS: A comparative study of gluconolactone versus benzoyl peroxide in the treatment of acne. Australas J Dermatol. 1992;33(3):131-4. Pubmed: 1303072
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- MacNeil ML, Steinberg KK, Yeager PR, Smith SJ: A semiautomated procedure for urinary D-glucaric acid using a centrifugal analyzer. J Anal Toxicol. 1986 Jan-Feb;10(1):15-7. Pubmed: 3951202
- Puri RN, Zhou FX, Colman RF, Colman RW: Plasmin-induced platelet aggregation is accompanied by cleavage of aggregin and indirectly mediated by calpain. Am J Physiol. 1990 Dec;259(6 Pt 1):C862-8. Pubmed: 2148055
- Rakotomanga S, Baillet A, Pellerin F, Baylocq-Ferrier D: Simultaneous determination of gluconolactone, galactonolactone and galactitol in urine by reversed-phase liquid chromatography: application to galactosemia. J Chromatogr. 1991 Oct 4;570(2):277-84. Pubmed: 1797843
- Steinberg KK, Needham LL: A comparison of two methods for quantifying D-glucaric acid. J Anal Toxicol. 1986 Jul-Aug;10(4):139-41. Pubmed: 3747452
- van Weely S, Brandsma M, Strijland A, Tager JM, Aerts JM: Demonstration of the existence of a second, non-lysosomal glucocerebrosidase that is not deficient in Gaucher disease. Biochim Biophys Acta. 1993 Mar 24;1181(1):55-62. Pubmed: 8457606
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|